Training with the HHS







Training with the U.S. Department of Health and Human Services
The U.S. Department of Health and Human Services offers a FREE Human Research Protection Foundational Training Program.
You can access Human Research Protection Foundational Training by navigating to the following website: https://www.hhs.gov/ohrp/education-and-outreach/online-education/human-research-protection-training/index.html
Additional free training opportunities are available on this page but are not required.
The HHS program includes five lessons and takes approximately 4-6 hours to complete. All lessons are based on the 2018 Revised Common Rule:
1. When HHS Regulations Apply (Lesson 1): This lesson introduces human research protections, the Common Rule, and the offices and agencies that oversee human subjects research. It takes approximately 35 min to complete.
2. What is Human Subjects Research (Lesson 2): This lesson details how the Common Rule regulations define “research” and “human subjects” and explains what it means to be exempt from the Common Rule regulatory requirements. It takes approximately 1 hr and 35 min to complete.
3. What are IRBs (Lesson 3): This lesson explains the purpose of and membership requirements for Institutional Review Boards, or IRBs. It takes approximately 45 min to complete.
4. IRB Review and Research (Lesson 4): This lesson describes the regulatory requirements for IRB review and the criteria for IRB review and approval under the Common Rule. It takes approximately 1 hour and 40 min to complete.
5. Institutional Oversight of Human Research (Lesson 5): This lesson explains the requirements for research institutions and IRBs for ensuring regulatory compliance and oversight of research to protect human participants. It takes approximately 45 min to complete.
Lessons can be completed separately from one another. There is no lesson timer, so users can take as long as they wish for each section. However, if you exit from the lesson page, your progress will not be saved. You should not exit the page until you have completed the lesson.
Once you have completed each lesson, you will be prompted to provide your name and completion date. You may then click “Print Certificate” and use “Print as PDF” to save the document. Please note that you will need to keep each certificate for your records, so make sure you have successfully saved each lesson’s certificate before moving on to the next lesson.
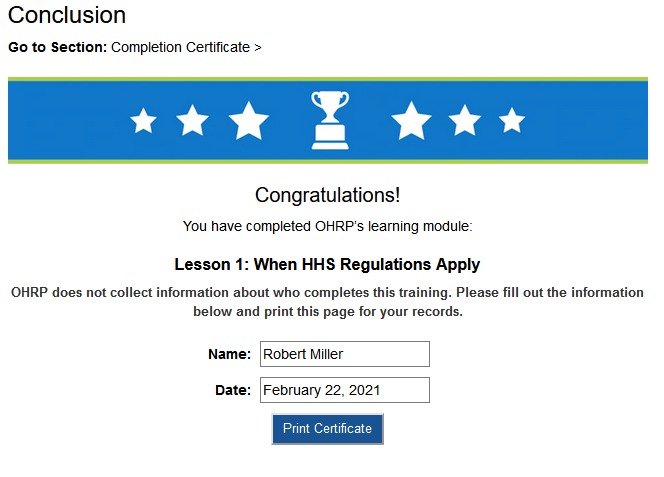
Figure 1. An example completion certificate. Once you have entered your name and the completion date, click “Print Certificate”. Select “Print as PDF” to save the certificate directly to your computer.
After you complete Human Research Protection Foundational Training with the U.S. Department of Health and Human Services, you should have 5 certificates of completion (one for each lesson). You must submit proof of completion (i.e., all 5 certificates) to have your training recognized and approved by Lyon College’s Institutional Review Board (IRB). You can find additional information about submitting proof of ethics training by navigating to the “Submit Proof of Completion” tab on the “Ethics Training” webpage (on the navigation bar).